Device Name: ibiomedi Electronic Stethoscope ES-2020
510(k) Number : K220466
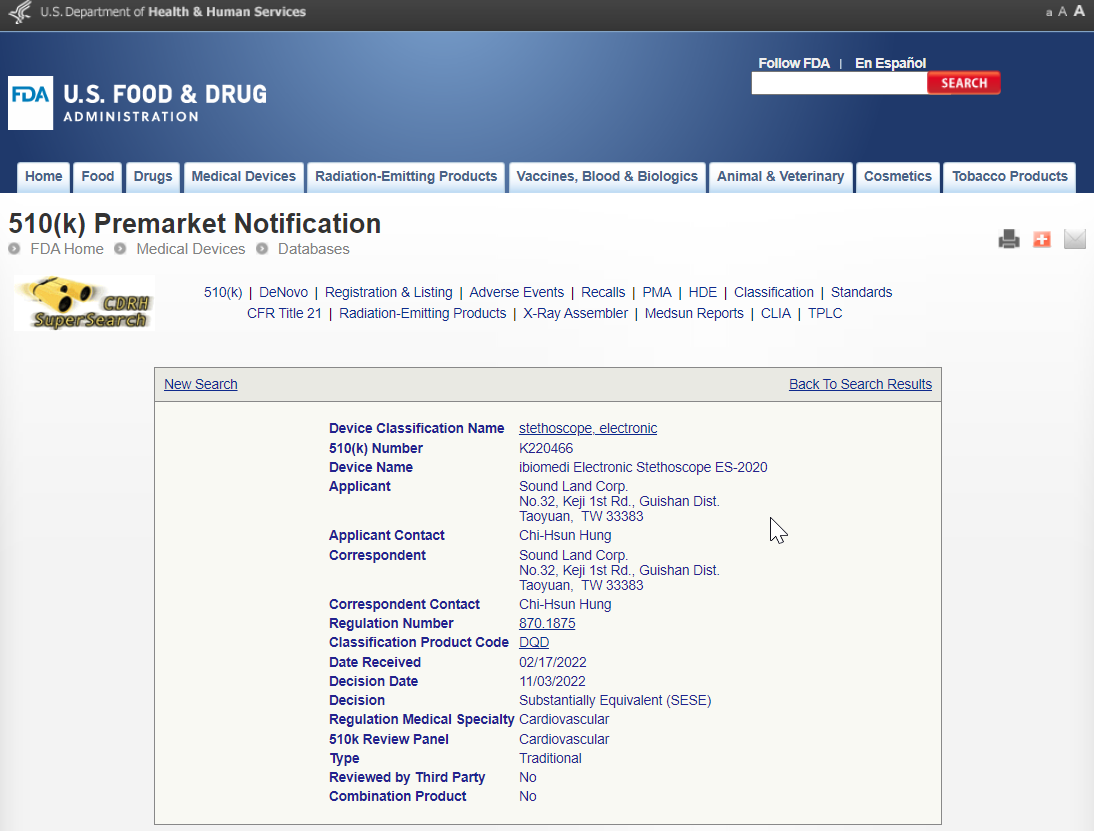
ibiomedi Electronic Stethoscope ES-2020 has obtained FDA certification.
FDA WEBSITE: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K220466
Device Name: ibiomedi Electronic Stethoscope ES-2020
510(k) Number : K220466
Device Name: ibiomedi Electronic Stethoscope ES-2020
510(k) Number : K220466







